Jesse Stoops, Dr Amit Patra & Geert Van de Mierop
Jesse Stoops, Dr Amit Patra & Geert Van de Mierop
Introduction
Endotoxins are potentially toxic compounds from bacterial origin. Once absorbed, endotoxins induce an inflammatory response, thus wasting energy and nutrients meant for growth and production. The most well-known endotoxins are lipopolysaccharides (LPS), which are a component of the cell wall of Gram-negative bacteria. In poultry, the gastrointestinal tract is the most important source of endotoxins and the main risk site where endotoxins can be transferred from the lumen into the bloodstream.
Nutrex developed a new innovative feed additive, EndoBan, by combining different strategies to reduce the leakage of endotoxins and improve animal’s performance.
The aim of this trial was to investigate the effect of EndoBan on growth performance and LPS absorption in broilers undergoing an intestinal challenge.
A pen trial was conducted in which Cobb 430Y male broilers were reared in a poultry house (AgriVet, India) for 42 days. A total of 198 broilers were randomly allocated to 3 treatments (Table 1) with 6 replicates per treatment (11 birds/pen at the start of trial). For all animals, a three phase dietary program (starter d0-14, grower d15-28 and finisher d29-42) was used in which all diets were fed ad libitum. The composition of the dietary diets is listed in Table 2.
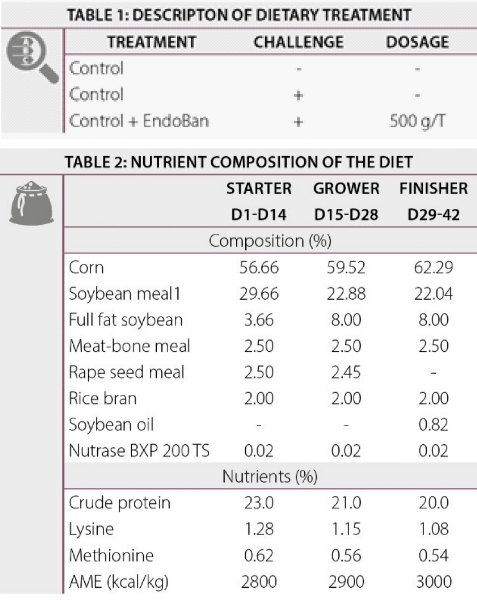
On day 17, birds from the challenge groups were administered with a coccidial suspension consisting of 10000 sporulated oocysts of Eimeria acervulina, Eimeria maxima and Eimeria tenella via oral gavage. After the coccidial treatment, a bacterial suspension consisting of E. coli (1010 cfu/bird) was given daily by oral gavage from day 21 till 22. At day 25, blood samples were taken (10 birds/treatment) to analyze the LPS concentration and the concentration of α-1-acid glycoprotein (AGP). A schematic presentation of the protocol is shown in Figure 1. Body weight and feed intake were recorded at weekly intervals. Feed conversion was calculated from the measured weight gain and feed intake. Pen mortality was recorded to correct feed intake.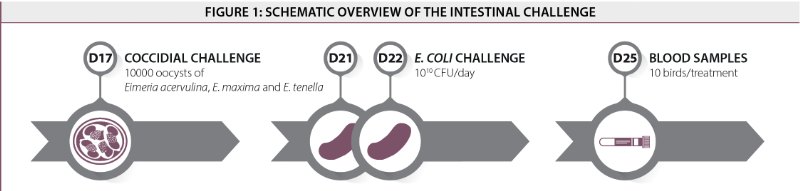
Results
The intestinal challenge resulted in reduced animal performance. At the end of the trial (day 42), the best results were obtained for the non-challenged control group. Nevertheless, challenged birds supplemented with EndoBan could alleviate the negative effects of the challenge and had an improved BW (+ 83g) compared to the challenged control group.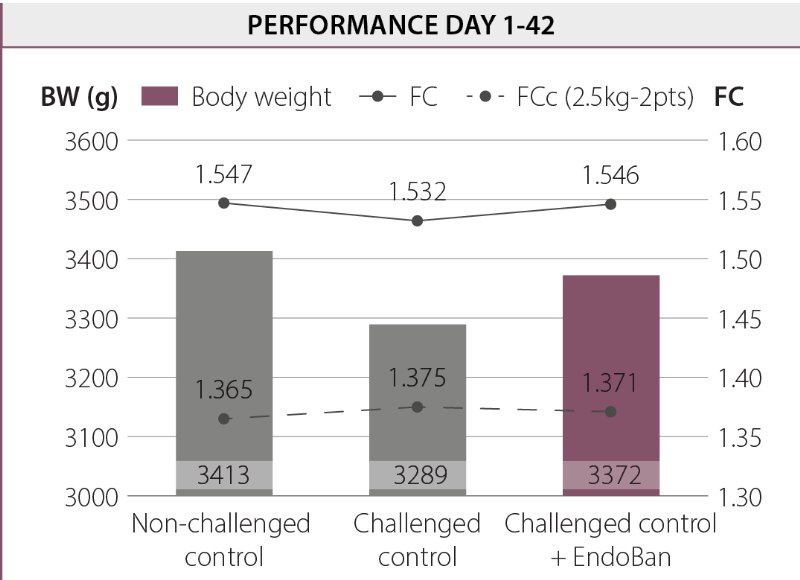
In this study, an oral coccidial and E. coli suspension were used to induce intestinal challenge. Any damage to the intestinal barrier could increase gut permeability and the translocation of endotoxins from the intestinal lumen into the bloodstream. The challenge used in this study led to a higher leakage of LPS molecules into the bloodstream and the production of acute phase proteins (e.g. AGP). Dietary supplementation of EndoBan decreased the LPS concentration in blood compared to the challenged control group and even the non-challenged control group. Moreover, birds fed the EndoBan supplemented diet had significantly (p < 0.01) lower serum AGP concentrations compared to the challenged control group.
CONCLUSIONS
The intestinal challenge used in this study was efficient to cause a disturbance in the intestinal barrier leading to reduced growth performance and higher LPS absorption.
EndoBan showed improved or similar responses as the non-challenged group, indicating that EndoBan neutralizes the negative effects caused by the challenge model. Therefore, we see that EndoBan can effectively reduce the translocation of endotoxins from the lumen into the bloodstream and improve animal’s performance.
 Agrinews24 কৃষির সাথে, কৃষকের পাশে
Agrinews24 কৃষির সাথে, কৃষকের পাশে